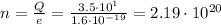Answer:
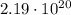 electrons
electrons
Step-by-step explanation:
I assume you mean:
How many electrons are there in
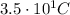 ?
?
Solution:
The charge of one electron is (in magnitude)
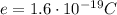
The charge in this problem is
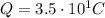
So, we can find how many electrons are in this charge by simply dividing the total charge by the charge of one electron: