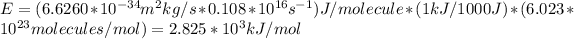Answer:
2.825*10^{3}kJ/mol
Step-by-step explanation:
We need to calcule the energy of a photon using Planks Formula:
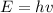
Where h is planks constant (6.62607004 × 10-34 m2 kg / s) and v is the frequency of the photon, the resulting energy will result in J/molecule if we want the energy in kJ/mol we need to divide by 1000 to change J into kJ and multiply by avogadros number: