Step-by-step explanation:
Relation between half-life and rate constant is as follows.
Half life
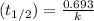
Hence, calculate the rate constant as follows.
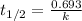
k =
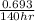
= 0.00495
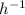
Hence, rate constant at
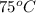 is 0.00495
is 0.00495
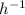 .
.
Now, at
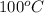 , value of rate constant will be as follows.
, value of rate constant will be as follows.
k =
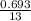
= 0.0533
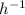
Hence, calculate the activation energy as follows.
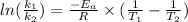
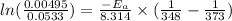
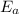 = 102.6 kJ/mol
= 102.6 kJ/mol
Therefore, value of activation energy is 102.6 kJ/mol .
Now, k at
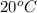 ,
,
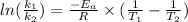
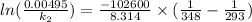
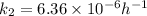
Calculate the time as follows.
kt =
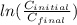
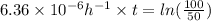
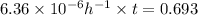
t = 108985 hours
Thus, we can conclude that time required for 50% hydrolysis of pyrophosphate is 108985 hours.