Step-by-step explanation:
The given data is as follows.
mass (m) = 107 g,
 =
=
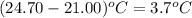
Specific heat of water = 4.18
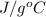
Since, the relation between heat energy, mass and temperature change is as follows.
q =
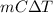
Hence, putting the given values into the above formula to calculate the heat energy as follows.
q =
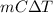
=
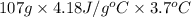
= 1654.86 J
Therefore, calculate the enthalpy of this reaction for 2.00 mol of a compound as follows.
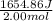
= 827.43 J/mol
or, =
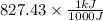 (as 1 kJ = 1000 J)
(as 1 kJ = 1000 J)
= 0.827 kJ/mol
Therefore, we can conclude that enthalpy of this reaction is 0.827 kJ/mol.