Answer:
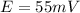
Step-by-step explanation:
Hello,
Considering the given information, the new concentration of Na+inside will be (13.6 + 4) mM = 17.6 mM, and outside will be (150 + 0.04) mM = 150.04 mM due to the previous specified conditions.
Now, the recalculation of the Nerst potential at the supposed temperature of 25 °C (which is modifiable) is done via:

Best regards.