Step-by-step explanation:
Formula to calculate final pressure is as follows.
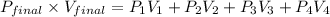 ............... (1)
............... (1)
Since, earlier the tube had no gas therefore, its pressure was zero initially considering temperature remains constant.
So, total number of moles will be as follows.
n =
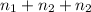
As, T and R will be common on both the sides. Hence, they will cancel out from both the sides.
Therefore, putting the given values into equation (1) as follows.
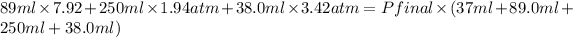
704.88 + 485.0 + 129.96 =
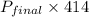
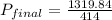
= 3.18 atm
Therefore, we can conclude that final pressure of the whole system in atm is 3.18 atm.