Answer:
Cyanohydrin is formed through nucleophilic addition of cyanide ion to carbonyl center of the given ketone
Step-by-step explanation:
- NaOH maintains basic medium. Hence dissociation of HCN gets favored to produce nucleophile
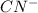
- In first step,
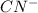 is produced through dissociation of HCN.
is produced through dissociation of HCN. - In second step,
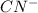 gives nucleophilic addition at the cabonyl center of heptan-4-one,
gives nucleophilic addition at the cabonyl center of heptan-4-one, - In the third step, protonation of negatively charged oxygen atom occurs leading to formation of cyanohydrin.
- Full reaction mechanism has been shown below.