Answer:
Given that
P = RT/V + a/V²
We know that
H= U + PV
For T= Constant (ΔU=0)
ΔH= ΔU +Δ( PV)
ΔH= Δ( PV)
P = RT/V + a/V²
P V= RT + a/V
dH/dV = d(RT + a/V)/dV
dH/dV = - a/V²
So the expression of dH/dV
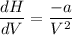
b)
In isothermal process
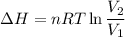 (ΔU=0)
(ΔU=0)
Now by putting the all values
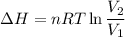
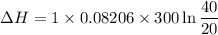
ΔH = 17.06 L.atm