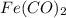Answer:
The empirical formula is =
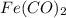
Step-by-step explanation:
Carbon dioxide obtained:
Pressure = 44.9 mm Hg
Also, P (mm Hg) = P (atm) / 760
Pressure = 44.9 / 760 = 0.0591 atm
Temperature = 25 °C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T₁ = (25 + 273.15) K = 298.15 K
V = 1.50 L
Using ideal gas equation as:
PV=nRT
where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 0.0821 L.atm/K.mol
Applying the equation as:
0.0591 atm × 1.50 L = n × 0.0821 L.atm/K.mol × 298.15 K
⇒n = 0.0036 moles
1 mole of carbon atoms are present in 1 mole of carbon dioxide. So,
Moles of C = 0.0036 moles
Molar mass of C atom = 12.0107 g/mol
Mass of C in molecule = 0.0036 x 12.0107 = 0.0432 g
Given that the compound only contains iron and carbon. So,
Mass of Fe in the sample = Total mass - Mass of C
Mass of the sample = 0.142 g
Mass of Fe in sample = 0.142 g - 0.0432 g = 0.0988 g
Molar mass of Fe = 55.845 g/mol
Moles of Fe = 0.0988 / 55.845 = 0.0018 moles
Taking the simplest ratio for Fe and C as:
0.0018 : 0.0036
= 1 : 2
The empirical formula is =