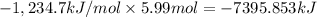Answer:
Enthaply change when 5.99 moles of alcohol undergone combustion is -7395.853 kilo Joules..
Step-by-step explanation:
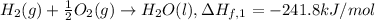 ..[1]
..[1]
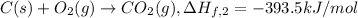 ..[2]
..[2]
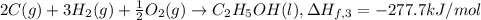 ..[3]
..[3]
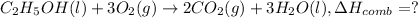 ..[4]
..[4]
2 × [2] + 3 × [1] - [3] = [4]
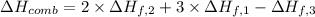
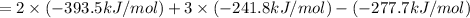
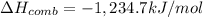
Enthalpy of combustion of ethanol is -1,234.7 kJ/mol.
Enthaply change when 5.99 moles of alcohol undergone combustion: