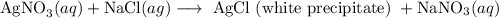The main products must be obtained during double displacement reaction are a precipitate and a gas
Option: A and B
Explanation:
"Double displacement reaction" is one of the types of chemical reactions. It is a process in which one component each of both the reacting molecules get exchanged to form the products which is precipitate obtained from mother liquor while rest is filtrate. Following is the reaction:
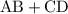
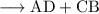
A Chemical bond responsible for holding the product as such may be covalent or ionic. Also called as "salt metathesis reaction" or double decomposition reaction. One of the examples are as follows where "aqueous solution" of silver nitrate is combined with sodium chloride and result in a curdy "white precipitate" of silver chloride: