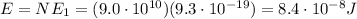Answer:
Step-by-step explanation:
The energy emitted by a single photon is given by:

where
h is the Planck constant
c is the speed of light
 is the wavelength of the photon
is the wavelength of the photon
For the photons emitted by the zinc atoms,
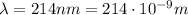
So the energy of a single photon emitted is

And since the number of atoms is
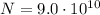
The total energy emitted will be