Answer:
mass conservation is valid for all closed system where the mass will remain in the system always
Step-by-step explanation:
Conservation of mass is applicable everywhere in classical physics
Here we can also apply mass conservation as we know that the initially the beaker and its water content is having total mass of 109.44 g
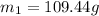
now when we heated it to higher temperature then its total mass will be lesser than the initial mass this is because some of the water may evaporate from the system.
Here if we repeat the same experiment with closed boundary then we can see that total mass will be conserved
So here mass conservation is valid for all closed system where the mass will remain in the system always