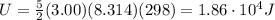Answer:
Step-by-step explanation:
I assume you mean
 , which is a diatomic gas.
, which is a diatomic gas.
For a diatomic gas, the internal energy is given by
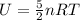
where
n is the number of moles
R is the gas constant
T is the absolute temperature
Here we have:
n = 3.00 mol
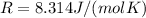
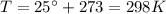
Substituting into the equation, we find the gas internal energy: