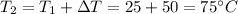Answer:
Step-by-step explanation:
When an amount of energy Q is supplied to a substance of mass m, the temperature of the substance increases by
 , according to the equation:
, according to the equation:
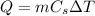
where
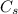 is the specific heat capacity of the substance
is the specific heat capacity of the substance
Here we have
m = 700.0 g of water
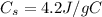 is the specific heat capacity of water
is the specific heat capacity of water
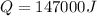 is the energy supplied
is the energy supplied
Solving for
 , we find that the temperature of the water increases by
, we find that the temperature of the water increases by
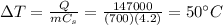
The initial temperature was
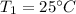
So the final temperature will be