Step-by-step explanation:
It is known that
 of propionic acid = 4.87
of propionic acid = 4.87
And, initial concentration of propionic acid =
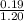
= 0.158 M
Concentration of sodium propionate = \frac{0.26}{1.20}[/tex]
= 0.216 M
Now, in the given situation only propionic acid and sodium propionate are present .
Hence, pH =
![pK_(a) + log(([salt])/([acid]))](https://img.qammunity.org/2020/formulas/chemistry/college/ppj10pki7g4vdpa9qddww0yjzpu032k2q3.png)
=
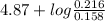
= 4.87 + log (1.36)
= 5.00
- Therefore, when 0.02 mol NaOH is added then,
Moles of propionic acid = 0.19 - 0.02
= 0.17 mol
Hence, concentration of propionic acid =
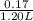
= 0.14 M
and, moles of sodium propionic acid = (0.26 + 0.02) mol
= 0.28 mol
Hence, concentration of sodium propionic acid will be calculated as follows.
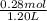
= 0.23 M
Therefore, calculate the pH upon addition of 0.02 mol of NaOH as follows.
pH =
![pK_(a) + log(([salt])/([acid]))](https://img.qammunity.org/2020/formulas/chemistry/college/ppj10pki7g4vdpa9qddww0yjzpu032k2q3.png)
=
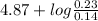
= 4.87 + log (1.64)
= 5.08
Hence, the pH of the buffer after the addition of 0.02 mol of NaOH is 5.08.
- Therefore, when 0.02 mol HI is added then,
Moles of propionic acid = 0.19 + 0.02
= 0.21 mol
Hence, concentration of propionic acid =
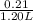
= 0.175 M
and, moles of sodium propionic acid = (0.26 - 0.02) mol
= 0.24 mol
Hence, concentration of sodium propionic acid will be calculated as follows.
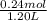
= 0.2 M
Therefore, calculate pH upon addition of 0.02 mol of HI as follows.
pH =
![pK_(a) + log(([salt])/([acid]))](https://img.qammunity.org/2020/formulas/chemistry/college/ppj10pki7g4vdpa9qddww0yjzpu032k2q3.png)
=
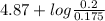
= 4.87 + log (0.114)
= 4.98
Hence, the pH of the buffer after the addition of 0.02 mol of HI is 4.98.