Answer:
Internal energy increases when heat is transferred to the system
Step-by-step explanation:
To answer this question you can make use of the first law of thermodynamics, which is a way to state the conservation of energy:
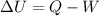
Where U is the internal energy of the system
Q is heat, and W is work done by the system.
This law tells us that the change in internal energy is proportional to the heat added to the system minus the work done by the system.
So for the internal energy of a system to increase, heat must be added to it.