Answer:
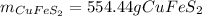
Step-by-step explanation:
Hello,
During this process, the following chemical reactions are carried out:
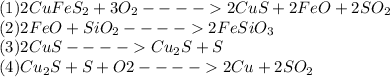
Now, a typical penny, has about 3.0 g of copper, it means that 100 pennies have 300 g of copper, thus, we develop the following stoichiometric procedure to determine the sulfur that is consumed in the 4th reaction, because in the third one it is produced:
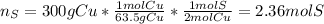
Since the percent yield for the second, third and fourth chemical reactions is 100%, we now proceed to the third reaction to compute consumed cupric sulfide since it is produced in the first chemical reaction, thus:
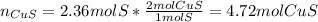
Finally, by using the first chemical reaction and the percent yield (represented by the by-moles relationship of cupric sulfide), one computes the required mass of chalcopyrite (
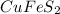 ) with a molecular mass of 183.54g/mol considering the first chemical reaction:
) with a molecular mass of 183.54g/mol considering the first chemical reaction:
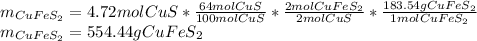
Best regards