Answer:
4.68x10⁹
Step-by-step explanation:
Kp is the equilibrium constant based on presure, and depends only on the gas substances. For a generic equation:
aA(g) + bB(g) ⇄ cC(g) + dD(g)
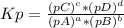
The reaction given can be summed to form the third one:
C(s) + CO₂(g) ⇄ 2CO (g) K'p = 1.30x10¹⁴
CO(g) + Cl₂(g) ⇄ COCl₂ (g) K''p = 6.00x10⁻³
We need to multiply the second reaction by 2, so CO will be simplified. If we multiplied a reaction for n, the new Kp will be (Kp)ⁿ, so:
C(s) + CO₂(g) ⇄ 2CO (g) K'p = 1.30x10¹⁴
2CO(g) + 2Cl₂(g) ⇄ 2COCl₂(g) (K''p)²= (6.00x10⁻³)²
The Kp of the reaction resulted by the sum will be: Kp = K'p*K''p
C(s) + CO₂(g) + 2Cl₂(g) ⇄ 2CO(g) + 2COCl₂(g)
Kp = 1.30x10¹⁴ * (6.00x10⁻³)²
Kp = 1.30x10¹⁴*3.60x10⁻⁵
Kp = 4.68x10⁹