Answer:
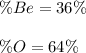
Step-by-step explanation:
Hello!
In this case, since the percent compositions of Be and O in beryllium oxide are computed as shown below:
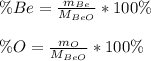
Since atomic masses are respectively 9.01 and 16.0 g/mol and the molar mass of the compound is 25.01 g/mol, the resulting percent compositions are:
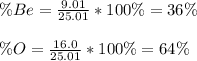
Best regards!