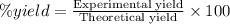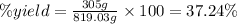Answer:
The percentage yield of the reaction is 37.24%.
Step-by-step explanation:
Experimental yield of nitrotoluene product = 305 g
Theoretical yield of nitrotoluene product = ?
Percentage yield:
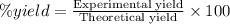
Theoretical yield of nitrotoluene product:
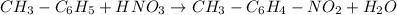
Moles of toluene =
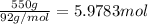
According to reaction 1 mole of toluene gives 1 mole of nitrotoluene product.
Then 5.9783 moles of toluene will give:
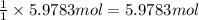 of nitrotoluene.
of nitrotoluene.
Mass of 5.9783 moles of nitrotoluene:
5.9783 mol × 137 g/mol = 819.03 g
Percentage yield of the reaction: