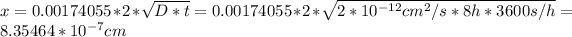Answer:
8.354 nanometers
Step-by-step explanation:
To treat a diffusive process in function of time and distance we need to solve 2nd Ficks Law. This a partial differential equation, with certain condition the solution looks like this:
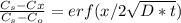
Where Cs is the concentration in the surface of the solid
Cx is the concentration at certain deep X
Co is the initial concentration of solute in the solid
and erf is the error function
Then we solve right side,

And we need to look up the inverse error function of 0.001964 resulting in: 0.00174055
Then we solve for x: