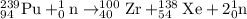Answer:
The particle which completes the given equation is :
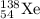
Step-by-step explanation:
The given reaction is of a fission reaction:
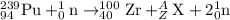
Total mass on the reactant side is equal to the total mass on the product side:
239 + 1 = 100 +A+ 2
A = 138
Sum of atomic numbers on the reactant side is equal to the sum of atomic number on the product side:
94 + 1(0) = 40 + Z + 2(0)
Z = 54
So atomic number 54 id of Xenon.
The particle which completes the given equation is :