Answer:
2,57 g of precipitate.
Step-by-step explanation:
For the reaction:
6 NaOH + Al₂(SO₄)₃ → 2 Al(OH)₃ + 3 Na₂SO₄
The precipitate is Al(OH)₃.
185,5mL of 0,533M NaOH are:
0,1855L × 0,533M = 0,0989 moles NaOH
Moles of Al₂(SO₄)₃ are:
15,8g ×
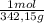 = 0,0462 moles Al₂(SO₄)₃
= 0,0462 moles Al₂(SO₄)₃
For the total reaction of 0,0989 moles NaOH with Al₂(SO₄)₃ you need:
0,0989moles NaOH ×
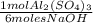 = 0,0165 moles Al₂(SO₄)₃
= 0,0165 moles Al₂(SO₄)₃
As you have 0,0462 moles Al₂(SO₄)₃ the limiting reactant is NaOH.
0,0989 moles of NaOH produce:
0,0989moles NaOH ×
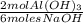 = 0,0330 moles of Al(OH)₃
= 0,0330 moles of Al(OH)₃
These moles are:
0,0330 moles of Al(OH)₃ × (78 g/mol) = 2,57 g of Al(OH)₃ ≡ mass of precipitate
I hope it helps!