Step-by-step explanation:
We know that 740 mm Hg = 0.97368 atm
According to ideal behavior for the vapor,
PV = nRT
here, R = 0.082 L
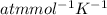
T = 273.15 + 62.7 = 335.85 K
V =
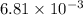 L
L
Therefore, first we will calculate the number of moles as follows.
n =
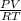
=
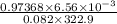
=
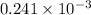 moles
moles
Now, molar mass of the given compound will be calculated as follows.
Number of moles =

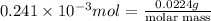
molar mass = 92.94 g/mol
Thus, we can conclude that molar mass of the compound is 92.94 g/mol.