Answer:
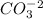 ,
,
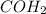 ,
,
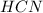 ,
,
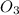
Step-by-step explanation:
In figure 1 we can several structures for
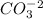 in which the double bond can be moved across the oxygens due to the resonance.
in which the double bond can be moved across the oxygens due to the resonance.
In figure 2 the double bond between the carbon and the oxygen in
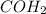 can be delocalized to the oxygen generating a new lewis structure in which we have a negative charge in the oxygen and a positive charge in the carbon.
can be delocalized to the oxygen generating a new lewis structure in which we have a negative charge in the oxygen and a positive charge in the carbon.
In figure 3 we have again a delocalization process upon the nitrogen in order to produce a negative charge in the nitrogen and a positive charge in the carbon generating a new lewis structure.
In figure 4 due to the resonance, the double bond to the central oxygen can be moved from left to right generating 2 lewis structures.
Finally in the two last molecules is not possible to move the electrons. For
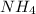 is not possible because there are no electrons to move. In the case of
is not possible because there are no electrons to move. In the case of
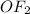 the movement of electrons would generate structures too unstable so it is not possible to generate more lewis structures.
the movement of electrons would generate structures too unstable so it is not possible to generate more lewis structures.