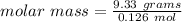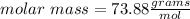Answer:
73.88 g/mol
Step-by-step explanation:
For this question we have to keep in mind that the unknown substance is a gas, therefore we can use the ideal gas law:

In this case we will have:
P= 1 atm
V= 3.16 L
T = 32 ªC = 305.15 ºK
R= 0.082
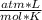
n= ?
So, we can solve for "n" (moles):
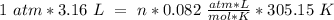
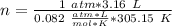
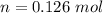
Now, we have to remember that the molar mass value has "g/mol" units. We already have the grams (9.33 g), so we have to divide by the moles: