The density of chloroform at 20°C is 1.4832 g/mL.
Density is a measure of mass per unit volume. It is typically expressed in units such as kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³). The formula for density is:
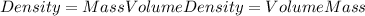
The density of chloroform can be calculated using the following equation:
Density = (mass of chloroform) / (volume of chloroform)
From the image, we can see that the mass of the chloroform is 145.10 g and the volume of the chloroform is 40.5 mL. Therefore, the density of chloroform is:
Density = (145.10 g) / (40.5 mL) = 3.58 g/mL
However, the image also states that the temperature is 20°C. At this temperature, the density of chloroform is known to be 1.4832 g/mL. Therefore, the answer to the question is:
The density of chloroform at 20°C is 1.4832 g/mL.