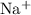Answer:
An ion is polyatomic if it contains more than one atoms.
Step-by-step explanation:
The prefix "poly-" in the word polyatomic means "more than one." A polyatomic ion contains more than one atoms. Note that these atoms might belong to the same element. For example, in the book Foundations of Introductory College Chemistry, the triiodide ion
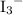 (note the subscript) is also classified as "polyatomic."
(note the subscript) is also classified as "polyatomic."
Other (more common) examples include:
- the sulfate ion,
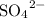 , which contains five atoms, and
, which contains five atoms, and - the hydroxide ion,
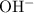 , which contains two atoms.
, which contains two atoms.
In contrast, an ion is monatomic (with the prefix "mono-") if it contains only one atom. Examples include:
- the chloride ion,
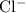 ,
, - the iodide ion,
 (without the subscript,) and
(without the subscript,) and - the sodium ion,