Answer:
-232 °C
Step-by-step explanation:
We can use the Ideal Gas Law and solve for T.
pV = nRT
Data:
m = 20.0 g
p = 6.0 atm
V = 0.40 L
R = 0.082 06 L·atm·K⁻¹mol⁻¹
Calculations:
1. Moles of N₂
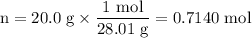
2. Temperature of N₂

3. Convert the temperature to Celsius
T = (41.0 - 273.15) °C = -232 °C