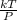Answer:
There will be No change in volume.
Step-by-step explanation:
Ideal Gas Equation is used to solve this :
PV=nRT;
P is pressure in Pa,
V is volume in
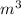 ,
,
n is number of moles of gas;
R is the universal gas constant, 8.31 J/K mol
T is temperature in Kelvin
when mass is said to be fixed, the number of moles will also be fixed, too. So combining both the constant term n and R which gives
PV=kT
where k is constant.
V=
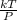
So the equation represents that, Volume V will remains unchanged when pressure and temperature are both doubled, therefore the numerator term T and the denominator term P are both gets affected by multiplication of 2, hence by cancellations:
V =
 =
=