Answer:
7 to 4 (higher
 )
)
5 to 3
3 to 2
4 to 2 (lower
 )
)
Step-by-step explanation:
We can use the Rydberg formula which relates the wavelength of the photon emissions to the principle quantum numbers involved in the transition:
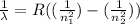
with
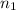 final n, and
final n, and
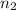 initial n
initial n
evaluating for each transition:
7 to 4
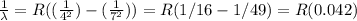
5 to 3
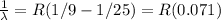
4 to 2
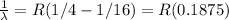
3 to 2
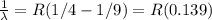
Note that the above formula is written for
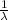 , so lower
, so lower
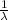 value obtained involves higher
value obtained involves higher
 .
.
So we should order from lower to higher
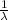
7 to 4 (higher
 )
)
5 to 3
3 to 2
4 to 2 (lower
 )
)
Note: Take into account that longer wavelength involves lower energy (
 ).
).