Answer:
IHD = 0
Step-by-step explanation:
Given that
C₆H₁₅N
Number of carbon atoms(n) = 6
Number of hydrogen atoms(x') = 15
Number of nitrogen atoms = 1
There is nitrogen atoms then x = x' -1
The index of hydrogen deficiency given as
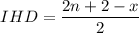
So
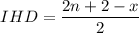
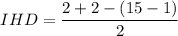
IHD = 0
The index of hydrogen deficiency is zero.