Answer:
The frequency of the photon that can dissociate dichlorine is 6.02×10¹⁴ Hz
Step-by-step explanation:
The energy of a photon is given by the equation:
E=h·f
E=3.99×10⁻¹⁹ J/molecule
h (Planck's constant)=6.626×10⁻³⁴ m²·kg/s
∴ f=E/h
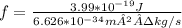 =6.02×10¹⁴ s⁻¹= 6.02×10¹⁴ Hz
=6.02×10¹⁴ s⁻¹= 6.02×10¹⁴ Hz