Answer:
2.41 M
Step-by-step explanation:
6Fe⁺²(aq) + BrO₃⁻(aq) + 6H⁺(aq) → 6Fe³⁺(aq) + Br⁻(aq) + 3H₂O(l)
- The molar concentration of Fe²⁺ (or [Fe²⁺] ) is equal to the moles of Fe²⁺ divided by the volume, which in this case is 11.00 mL.
- The moles of Fe²⁺ are calculated from the moles of BrO₃⁻:
0.118 M KBrO₃ * 37.50 mL = 4.425 mmol BrO₃⁻
4.425 mmol BrO₃⁻ *
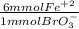 = 26.55 mmol Fe²⁺
= 26.55 mmol Fe²⁺
Thus [Fe²⁺] = 26.55 mmol Fe²⁺ / 11.00 mL = 2.41 M