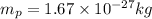Answer:
B) Within an atom, an electron can have only particular energies.
Step-by-step explanation:
As we know that electrons have energy but apart from electrons we know that protons and neutrons inside the nucleus of atom will also have energy in them.
rest all the statements are true as we have
A) Electrons orbit the nucleus rather like planets orbiting the Sun.
TRUE, because electrons can move in stationary orbit around the nucleus
C) Electrons can jump between energy levels in an atom only if they receive or give up an amount of energy equal to the difference in energy between the energy levels.
Difference amount of energy is lost or absorbed by the electron in form of photons
D) An electron has a negative electrical charge.
Charge of an electron is given as
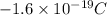
E) Electrons have very little mass compared to protons or neutrons
Mass of an electron is given as
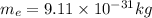
mass of proton or neutron