Answer:
Molarity of NaOH solution is 1.009 M
Step-by-step explanation:
Molar mass of HCl is 36.46 g/mol
Number moles = (mass)/(molar mass)
So, 0.8115 g of HCl =
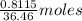 HCl = 0.02226 moles HCl
HCl = 0.02226 moles HCl
1 mol of NaOH neutralizes 1 mol of HCl.
So, if molarity of NaOH solution is S(M) then moles of NaOH required to reach endpoint is
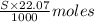
So,
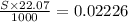
or, S = 1.009
So, molarity of NaOH solution is 1.009 M