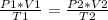Answer:
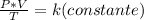
Or when comparing two conditions:
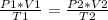
Step-by-step explanation:
Gas laws are a set of chemical and physical laws that allow determining the behavior of gases in a closed system. The parameters evaluated in these laws are pressure, volume, temperature and moles.
As the volume increases, the particles (atoms or molecules) of the gas take longer to reach the walls of the container and therefore collide less times per unit of time against them. This means that the pressure will be lower because it represents the frequency of gas shocks against the walls. In this way the pressure and volume are related, determining Boyle's law that says:
"The volume occupied by a certain gas mass at a constant temperature is inversely proportional to the pressure"
Boyle's law is expressed mathematically as:
Pressure * Volume = constant
or P * V = k
It is now possible to assume that you have a certain volume of V1 gas that is at a pressure P1 at the beginning of the experiment. If you vary the volume of gas to a new V2 value, then the pressure will change to P2, and the following will be true:
P1 * V1 = P2 * V2
Gay-Lussac's law can be expressed mathematically as follows:
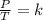
Where V = volume, T = temperature, K = Constant
This law indicates that the ratio between pressure and temperature is constant.
This law indicates that, as long as the volume of the container containing the gas is constant, as the temperature increases, the gas molecules move faster. Then the number of shocks against the walls increases, that is, the pressure increases. That is, the gas pressure is directly proportional to its temperature.
In short, when there is a constant volume, as the temperature increases, the gas pressure increases. And when the temperature decreases, gas pressure decreases.
Having two gases in different states it is possible to say:
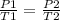
Finally, Charles's Law consists in the relationship between the volume and temperature of a certain amount of ideal gas, which is maintained at a constant pressure, by means of a constant of proportionality that is applied directly. For a given sum of gas at a constant pressure, as the temperature increases, the volume of the gas increases and when the temperature decreases, the volume of the gas decreases because the temperature is directly related to the energy of the movement of the gas molecules .
In summary, Charles's law is a law that says that when the amount of gas and pressure remain constant, the ratio between volume and temperature will always have the same value:
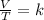
To compare two volume-temperature conditions for a given sample of a constant pressure gas:
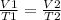
Combined law equation is the combination of three gas laws called Boyle's, Charlie's and Gay-Lusac's law. And this law examines the behavior of a constant amount of gas when pressure, volume and/or temperature is allowed to change.
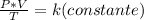
Or when comparing two conditions: