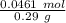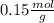Answer:
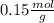
Step-by-step explanation:
The first step is to calculate the total amount of moles of HCl, so:
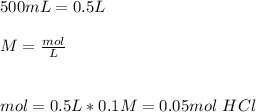
Then we can calculate the moles of HCl consumed in the reaction of NaOH:
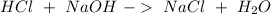
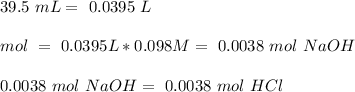
Then we have to substract from the intial value to obtain the moles of HCl that react with the sample:
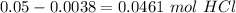
Finally to know the ratio between grams and moles we have to divide: