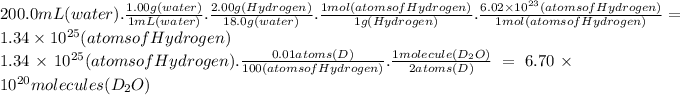Answer:
6.70 × 10²⁰ molecules of D₂O
Step-by-step explanation:
The number of D₂O molecules is related to the abundance of deuterium. If the word "Hydrogen" refers to both isotopes, while H and D refer to each one.
We will use these relations:
The molar mass of water is 18.0g/mol
The molar mass of Hydrogen is 1.00 g/mol
Avogadro's number is 6.02 × 10²³
Every 100 atoms of Hydrogen, there are 0.01 atoms of D.
We can pose the following proportions: