Answer:
methyl tert-butyl ether is produced as a product
Step-by-step explanation:
- Nucleophilic substitution in solvolysis reaction of tert-butyl bromide goes through
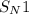 mechanism.
mechanism. - Because Br is a good leaving group due to it's large size and high polarizability and methanol is an weak nucleophile.
- In the first step, tert-butyl cation is produced. In the second step, methanol attacks the carbocation and produce methyl tert-butyl ether after deprotonation.
- Reaction and structure of product has been shown below.