Answer:
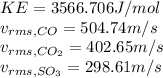
Step-by-step explanation:
Hello,
Kinetic energy is computed for all the gases as follows, since it does not depend on a particular property of the gas:
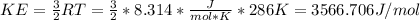
On the other hand, root mean square velocities for each compound depend on their molecular mass and are computed as follows:

Best regards.