Answer:
Calcium hydroxide.
Step-by-step explanation:
The gastric juice is a solution of hydrochloric acid. It is highly acidic. Antacids contain basic chemicals that could neutralize that acid.
If the active ingredient in an antacid medication is a metal hydroxides ("alkalis,") it is likely to be calcium hydroxide
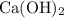 . Compared with other metal hydroxides,
. Compared with other metal hydroxides,
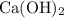 is superior in multiple ways.
is superior in multiple ways.
Calcium hydroxide is less soluble and is a relative mild metal hydroxide
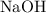 and
and
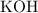 rapidly dissolve in water to produce a strongly basic solution. In contrast, the solubility of calcium hydroxide in pure water is relatively low. That means that there's a lower chance for antacids based on calcium hydroxide to irritate the mouth and throat while being swallowed.
rapidly dissolve in water to produce a strongly basic solution. In contrast, the solubility of calcium hydroxide in pure water is relatively low. That means that there's a lower chance for antacids based on calcium hydroxide to irritate the mouth and throat while being swallowed.
Calcium hydroxide is generally non-toxic
Hydroxides of toxic "heavy" transition metals, such as copper (II) hydroxide, are even weaker than calcium hydroxide. However, when these alkalis react with the HCl in gastric juice, they release their toxic metal cations. As a result, these alkali are seldom used in antacids. In contrast, calcium occurs naturally in many dairy products and is not as toxic.
Magnesium hydroxide
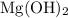 is weaker than calcium hydroxide and is also not toxic. However, since the magnesium ion
is weaker than calcium hydroxide and is also not toxic. However, since the magnesium ion
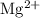 is a potential laxative, it is not as frequently used as calcium hydroxide in antacids.
is a potential laxative, it is not as frequently used as calcium hydroxide in antacids.