Answer:
181.90 mmHg
Step-by-step explanation:
- According to pressure law, the pressure of a gas and its absolute temperature are directly proportional at a constant volume.
- Mathematically,
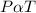
- Thus;
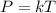
- Therefore, at varying temperature and pressure;
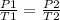
In this case, we are given;
Initial temperature, T1 = 77 K
Initial pressure, P1 = 47 mmHg
Final temperature, T2 = 25 °C + 273
= 298 K
We are required to find the final pressure;
Using pressure law;
P2 = (P1/T1)T2
= (47/77K) × 298 K
= 181.90 mmHg
Therefore, the new pressure will be 181.90 mmHg