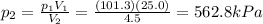Answer:
562.8 kPa
Step-by-step explanation:
We can solve the problem by using Boyle's law, which states that the product between the pressure of a gas kept at constant temperature and its volume is constant:
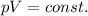
which can also be written as
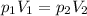
In this problem:
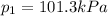 is the initial pressure of the gas
is the initial pressure of the gas
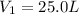 is the initial volume
is the initial volume
Later, the gas is pumped into a volume of
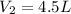
So we can use the equation above to find
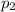 , the final pressure of the gas when it is inside the ball:
, the final pressure of the gas when it is inside the ball: