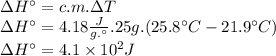Answer:
The change in enthalpy is 4.1 × 10² J.
Step-by-step explanation:
We can calculate the change in enthaply (ΔH°) using the following equation:
ΔH° = c . m . ΔT [1]
where,
c is the specific heat capacity of the solution
m is the mass of the solution
ΔT is the change in temperature (Tfinal - Tinitial)
We can calculate the mass, from the density formula:
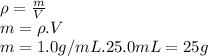
If we replace this data in equation [1]: