Answer:
The specific rotation is 62 °.mL . dm⁻¹ g⁻¹.
Step-by-step explanation:
In a polarimetric experiment, we can calculate the specific rotation (
![[\alpha ]_(D)^(20)](https://img.qammunity.org/2020/formulas/chemistry/college/7mnsck2lts2c41g9wqygjqoxnug0j425hh.png) ) for ecdysone using the following expression:
) for ecdysone using the following expression:
![[\alpha ]_(D)^(20) =(\alpha )/(l * c )](https://img.qammunity.org/2020/formulas/chemistry/college/dviq55dmoivmmqsodytl4vq7kz8ei88v5n.png)
where,
α is the angle of rotation
l is the path length in decimeters (2.0 cm = 0.20 dm)
c is the concentration in g/mL
Concentration is:
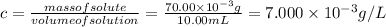
Then,
![[\alpha ]_(D)^(20) =(\alpha )/(l * c )=(0.087\textdegree )/(0.20dm * 7.000 * 10^(-3)g/mL ) =62 \textdegree .mL .dm^(-1). g^(-1)](https://img.qammunity.org/2020/formulas/chemistry/college/ucft97xrfdku1caie2anrl7spjbbhjiq4r.png)