Answer:
The diatomic element in the syringe is O₂.
Step-by-step explanation:
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of the gases:
P*V=n*R*T
Taking into account the following values:
- P=2.33 atm
- V= 45 mL= 0.045 L (1 L= 1,000 mL)
- R= 0.082
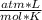
It is possible to calculate the amount of moles of gas present in the syringe as:
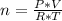
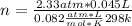
n= 0.0043 moles.
The mass of the syringe increases by 0.137 g when filled. This indicates that the 0.0043 moles calculated previously contain a mass of 0.137 g. Then it must be verified for each diatomic gas presented in the options that the 0.0043 moles contain said amount of mass. For this, the molar mass of each compound is known:
- H₂: 2 g/mol
- O₂: 32 g/mol
- F₂: 38 g/mol
Now a rule of three applies for each case as follows:
- if 1 mole of H₂ contains 2 g of the gas, 0.0043 moles of H₂, how much mass will it contain?
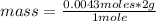
mass= 0.0086 g
- if 1 mole of O₂ contains 32 g of the gas, 0.0043 moles of O₂, how much mass will it contain?
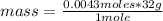
mass= 0.137 g
- if 1 mole of F₂ contains 38 g of the gas, 0.0043 moles of F₂, how much mass will it contain?
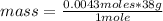
mass= 0.163 g
0.0043 moles contain a mass of 0.137 g in the case of O₂
The diatomic element in the syringe is O₂.