Answer:
In the original sample there's 72.78% KCl
Step-by-step explanation:
KCl(aq) + AgNO₃(aq) → AgCl(s) + KNO₃(aq)
- To solve this problem we need to calculate the mass of pure potasssium chloride. To do that we calculate the moles of KCl that reacted into AgCl:
1.26 g AgCl *
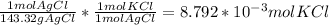
- Now with the molecular weight of KCl, we can calculate the mass of KCl that reacted:
8.792 * 10⁻³ mol KCl * 74.55 g/mol = 0.655 g KCl
- Finally we divide the mass of pure KCl by the mass of the sample, to calculate the percentage KCl:
0.655 / 0.900 * 100% = 72.78%