3 atoms of oxygen are there in 4H2PO3
Step-by-step explanation:
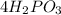 exists as an anion. 4H2PO3
exists as an anion. 4H2PO3
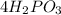 is s conjugate base of a phosphorous acid and conjugate acid of a the hydrogenphosphite.
is s conjugate base of a phosphorous acid and conjugate acid of a the hydrogenphosphite.
While representing any chemical compound ,numeric value that is suffixed before atom symbol denotes the number of atoms in that particular compound. In the equation
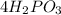 , there are 4 of Hydrogen (H) atoms. Two of Phosphorus Monoxide (PO) atom. And 3 of oxygen (O) atoms.
, there are 4 of Hydrogen (H) atoms. Two of Phosphorus Monoxide (PO) atom. And 3 of oxygen (O) atoms.
Thus it can be concluded that there are 3 oxygen atom present in the DihydrogenPhosphate